|
Molecular Magnetism; Creating the Objects of Study The preparation of Polynuclear Coordination Complexes of Transition Metals has been a primary goal in the area of Molecular Magnetism for a long time. A very prolific activity on this topic along with synergistic efforts by chemists and physicists have allowed the discovery of new phenomena such as Single-Molecule Magnetism or Quantum Tunneling in Magnetic Clusters. This has encouraged synthetic chemists to produce novel systems as potential objects of study of these or new properties. Design of Ligands for the Assembly of New Clusters A promising strategy for the synthesis of Transition Metal Aggregates with unprecedented geometry and properties is the design and preparation of novel polynucleating ligands with a specific disposition of donor atoms in their structure. We have developed a program aimed at producing ligands that incorporate a number of b-diketone moieties into their backbone, in addition to other donors, in order to produce arrays of transition metals arranged in modes that would not be observed otherwise. |
|
Dr. Guillem Aromí |
|
Research |
|
H3L1 |
|
H4L2 |
|
H5L3 |
|
Fig. 1 Some of the ligands prepared |
|
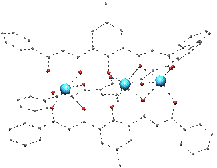
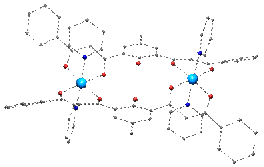
Reactions of ligands such as these in Figure 1 with 3d metal cations have produced a large variety of polynuclear compounds with novel topologies and nuclearities ranging two to infinite. Solvent Controlled Switch Ligand H3L1 has allowed the formation very rare asymmetric trinuclear helicates of CoII or MnII that can be reversibly converted into dinuclear complexes by changing the solvent. |
|
Synthetic Commun. 2003, 33, 11 Dalton Trans. 2004, 3586 |
|
pyridine |
|
CH2Cl2 |
|
[M3(HL1)3] |
|
[M2(HL1)2(py)4] |
|
Angew. Chem. Int. Ed. 2001, 40, 3444 Inorg. Chem. 2002, 41, 3673 |
|
Assembly of Metallic Chains The structure of H3L1 leads to the assembly of chains of four closely spaced metals under basic conditions. These dimerize into octanuclear clusters by the bridging action of methoxide ligands. Anions such as NO3-, Cl- or Br– link the clusters into 1-D coordination polymers. |
|
[Cu8(L1)2(OMe)8(NO3)2] |
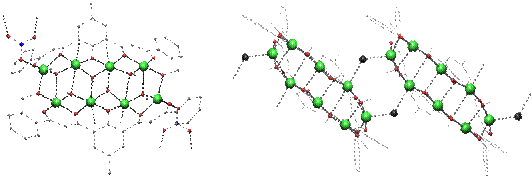
|
[Cu8(L1)2(OMe)8(X)2]; X=Cl, Br |
|
Angew. Chem. Int. Ed. 2002, 40, 1168 Chem., Eur. J. 2004, 10, 6476 |
|
Characterization Techniques The synthesis of ligands and coordination compounds is carried out for the most part at the laboratory of the Grup d’Interaccions Magnètiques (Universitat de Barcelona). These constitute modern new facilities, opened for use in January 2005. The group enjoys full access to the facilities of the NMR department of the Universitat de Barcelona (Unitat de Resonància Magnètica Nuclear) for rutine proton NMR, paramagnetic NMR or multinuclear NMR. Magnetic characterization is performed with the recently acquired Quantum Design SQUID magnetometer and the X-Band EPR spectrometer. All the routine techniques of characterization (IR, MS. Elemental Analyses, X-Ray Powder Diffraction, X-Ray Crystallography) are available from the various departments of the university. |
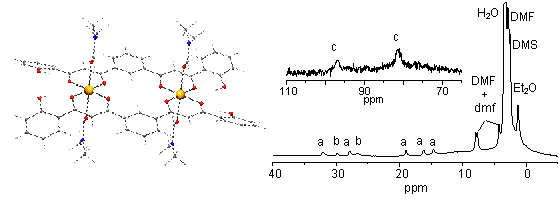
|
Proton Paramagnetic NMR of [Co2(H2L2)2(dmf)4] |
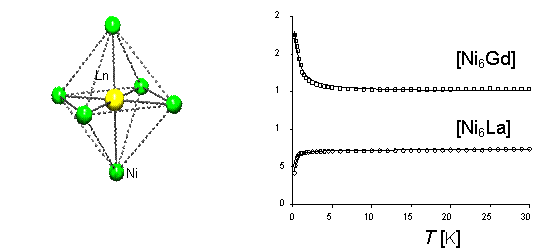
|
Magnetic Susceptibility of (NMe4)[Ni6Ln(pro)12](ClO4)4 (Ln=La3+, Gd3+) |
|
Dalton Trans. 2004, 3586 |
|
Angew. Chem. Int. Ed. 2005, 44, 1997 |